

![]()
![]()
![]()
![]()
![]()
![]()
![]() EDUCATION EXHIBIT
EDUCATION EXHIBIT
Balloon Dilation and
Stent Placement for
Esophageal Lesions:
Indications, Methods,
and Results
Eric Therasse, MD Vincent L. Oliva, MD Edwin Lafontaine, MD
ONLINE-ONLY
Pierre Perreault, MD Marie-France Giroux, MD Gilles Soulez, MD
CME
See www.rsna
.org/education
/rg_cme.html.
LEARNING
OBJECTIVES
After reading this
article and taking
the test, the reader
will be able to:
Discuss the indica-
tions for and results
of esophageal stent
placement.
Describe the inser-
tion techniques, ad-
vantages, and limita-
tions associated with
commercially avail-
able stents.
Recognize and
manage complica-
tions that may occur
following esophageal
stent placement.
Esophageal balloon dilation and expandable stent placement are safe,
minimally invasive, effective treatments for esophageal strictures and
fistulas. These procedures have brought the management of dysphagia
due to esophageal strictures into the field of interventional radiology.
Esophageal dilation is usually indicated for benign stenoses and is
technically successful in more than 90% of cases. Most patients with
esophageal carcinoma are not candidates for resection; thus, the main
focus of treatment is palliation of malignant dysphagia and esophagores-
piratory fistulas. Esophageal stent placement, which is approved only
for malignant strictures, is one of the main therapeutic options in af-
fected patients and relieves dysphagia in approximately 90% of cases.
Dedicated commercially available devices continue to evolve, each with
its own advantages and limitations. Stent placement is subject to tech-
nical pitfalls, and adverse events occur following esophageal proce-
dures in a minority of cases. Although chest pain is common and self-
limited, reflux esophagitis, stent migration, tracheal compression, and
esophageal perforation and obstruction require specific interventions.
In many cases, these complications can be recognized and treated by
the interventional radiologist with minimally invasive techniques.
RSNA, 2003
Abbreviation: FDA Food and Drug Administration
Index terms: Esophagus, diseases, 71.291, 71.321, 71.33 Esophagus, grafts and prostheses, 71.1269 Esophagus, interventional procedures,
Esophagus, neoplasms, 71.30 Esophagus, stenosis or obstruction, 71.74 Stents and prostheses, 71.1269
RadioGraphics 2003; Published online 10.1148/rg.231025051
From the Departments of Radiology (E.T., V.L.O., P.P., M.F.G., G.S.) and Surgery (E.L.), Centre Hospitalier de l’Universite´ de Montre´al
(CHUM), 3840 St Urbain St, Montreal, Quebec, Canada H2W 1T8. Recipient of a Certificate of Merit award for an education exhibit at the 2001
RSNA scientific assembly. Received March 8, 2002; revision requested April 25 and received May 24; accepted May 28. Address correspondence
to E.T. (e-mail: [email protected]
RSNA, 2003
![]()

![]()
![]()
![]() January-February 2003
January-February 2003
Introduction
Most cases of malignant dysphagia are due to
esophageal carcinomas that are unresectable at
presentation and for which palliation will be the
focus of treatment (1). Dysphagia has long been
treated with surgical and endoscopic interven-
tions. Esophageal bougienage may be performed
without guidance, and a rigid plastic stent could
be inserted endoscopically with or without fluoro-
scopic guidance. However, crossing tight esopha-
geal stenoses and positioning metallic stents are
sometimes difficult or impossible without fluoro-
scopic guidance and appropriate catheter technol-
ogy. Radiologists, who are already accustomed to
using similar materials and techniques for vascu-
lar and nonvascular interventions, are in the ideal
position to perform these interventions safely and
relatively easily. Esophageal procedures should be
adequately tailored to the underlying disease, and
one should take advantage of the characteristics
of various devices in specific situations. Although
many benign strictures can be treated with bal-
loon dilation, esophageal stent placement will be
one of the main palliative options in malignant
dysphagia. In addition, the radiologist should be
aware of possible complications of esophageal
interventions because in many cases he or she will
be able to prevent or treat these complications.
In this article, we review the indications for
and the methods and outcomes of balloon dila-
tion and stent placement for esophageal stric-
tures. We also describe various commercially
available stents as well as the advantages and limi-
tations of each stent. In addition, we discuss spe-
cific situations that require careful attention and
the management of possible complications of
stent insertion.
Indications
Esophageal dilation is usually indicated for be-
nign stenoses such as rings, webs, achalasia, and
strictures caused by peptic, postsurgical, postscle-
rotherapeutic, or corrosive injury (2). Dilation of
extrinsic esophageal compression is usually inef-
fective. In malignant lesions, dilation is some-
times performed prior to stent insertion or can be
used as a temporizing measure prior to surgery or
radiation therapy, but it is generally ineffective if
used alone.
RG f Volume 23 ● Number 1
Figure 1. Photographs show FDA-approved
covered esophageal stents: the Ultraflex stent (a),
the Wallstent II (b), and the Z-stent without an-
chors (shown with an antireflux valve [arrows])
(c)
Esophageal stents have been approved by the
U.S. Food and Drug Administration (FDA) only
for malignant strictures, whether intrinsic (esoph-
ageal carcinoma) or extrinsic (eg, lung carci-
noma). The use of stents in benign lesions is
plagued by a high long-term complication rate
and leads to tissue hyperplasia with recurrent
esophageal stenosis (3). However, good results
have been reported in benign lesions with retriev-
able stents that are left in the esophagus for a few
months (4). Covered metallic stent placement is
now the primary treatment for malignant bron-
choesophageal fistulas and is also accepted as the
main treatment option for malignant dysphagia in
patients with poor functional status who cannot
tolerate radiation therapy or chemotherapy, who
have advanced metastatic disease, or in whom
previous therapy has failed (5).
FDA-approved Stents
There are currently three self-expandable esopha-
geal stents approved by the FDA (Fig 1), and
their characteristics are listed in Table 1 (1). All
currently available esophageal stents are covered
to prevent tumor ingrowth and allow treatment of
fistulas, whereas their extremities are uncoated to
permit anchoring and prevent migration. These
stents have now completely replaced rigid plastic
tubes, which were associated with a high proce-
dural complication rate and poor palliation (5).
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]() RG f Volume 23 ● Number 1
RG f Volume 23 ● Number 1
Therasse et al 91
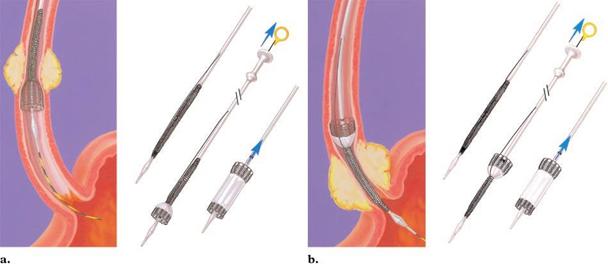
Figure 2. Drawings illustrate the delivery system of the Ultraflex stent. (a) The distal release system allows elimi-
nation of proximal foreshortening during deployment and is best suited for stent placement in the middle and upper
esophagus. (b) The proximal release system allows elimination of distal foreshortening during deployment and is best
suited for stent placement in the gastroesophageal junction. Arrows indicate how to release the stent by pulling out
the string. (Courtesy of Microvasive/Boston Scientific, Natick, Mass.)
Table 1
Characteristics of Covered Metallic Esophageal Stents
Feature
Deployment
Size of delivery system
Material
Design
Radial force
Edges
Lumen diameter (mm)
Length (cm)
Shortening (% of length)
Ultraflex*
String release
24-F
Nitinol
Knitted
Low
Uncoated, smooth
Wallstent II*
Sheath, pusher rod
18-F
Stainless steel
Mesh
High
Uncoated, sharp
Z-stent
Sheath, pusher rod
24–31-F
Stainless steel
Zigzag
Moderate
Coated, smooth
*Microvasive/Boston Scientific, Natick, Mass.
Wilson-Cook Medical, Winston-Salem, NC.
Ultraflex Stent
The Ultraflex stent is mounted on a delivery cath-
eter, unprotected by a sheath, and restrained by a
string that must be pulled out to release the stent.
Because of the stent shortening that occurs during
deployment, two delivery systems are proposed
(Fig 2). Preinsertion dilation is often required
because the unprotected surface of the stent and
the large profile of the delivery system produce
friction during advancement through tight esoph-
ageal strictures. Lubrication of the Ultraflex stent
delivery system is recommended to help with in-
sertion. Stent shortening, the poor radiopacity of
nitinol, and the need to predilate tight strictures
make Ultraflex stent deployment more difficult
than deployment of the Wallstent. The lower ra-
dial force and greater flexibility of the Ultraflex
stent are probably responsible for less postdeploy-
ment chest pain and better patient tolerance;
however, the Ultraflex stent also requires more
time to fully expand in comparison with other
stents (6). Smooth, nontraumatic edges should
offer protection against esophageal erosions and
bleeding. The Ultraflex stent is also available in a
22–23-mm-diameter version for use in dilated
esophagus.
![]()


![]()
![]()
![]() January-February 2003
January-February 2003
Figure 3. Photographs illustrate the de-
livery system of the Wallstent. (a) Wall-
stent before deployment. (b, c) To deploy
the stent, the inner catheter (arrow in b) is
held stationary while the outer sheath (ar-
rowhead in b) is withdrawn, allowing distal
to proximal stent release (c).
Wallstent
The Wallstent is mounted on a delivery catheter
and covered by a sheath. Stent deployment is il-
lustrated in Figure 3. Stent shortening occurs at
both extremities and must be considered when
choosing the appropriate stent length. The Wall-
stent is easy to deploy and is loaded in a small
RG f Volume 23 Number 1
(18-F) sheath that allows crossing of most lesions
without preinsertion dilation. The radiopacity of
the stent is excellent, and deployment requires
minimal traction on the sheath. The uncoated
sharp filaments of the stent extremities can be
traumatic but should reduce the occurrence of
migration. The Wallstent has the highest radial
force (more appropriate for extrinsic compres-
sion) of the FDA-approved stents and is more
likely to produce postdeployment chest pain.
Z-stent
The Z-stent must be inserted into the delivery
system before use (Fig 4). The Z-stent has good
radiopacity, and its shortening is minimal. How-
ever, considerable traction must be applied on the
outer tube to release the stent from the bulky
31-F sheath, which often requires preinsertion
dilation to 12 mm. In addition, removal of the
delivery system is often difficult because its tip has
a tendency to catch the stent. The Z-stent has
minimal flexibility, making it less appropriate for
tortuous anatomic structures. The stent is avail-
able with uncoated flange ends without anchors
(31-F) and with coated flange ends with anchors
(24-F). The former version is also available with
an antireflux valve to be used when the stent has
to cross the gastroesophageal junction. This
windsock-type valve, an extension of the stent
polyurethane membrane 8 cm beyond the lower
metal cage, is designed to invert inside the stent
![]()
![]()
![]()
![]() RG f Volume 23 ● Number 1
RG f Volume 23 ● Number 1
Therasse et al 93
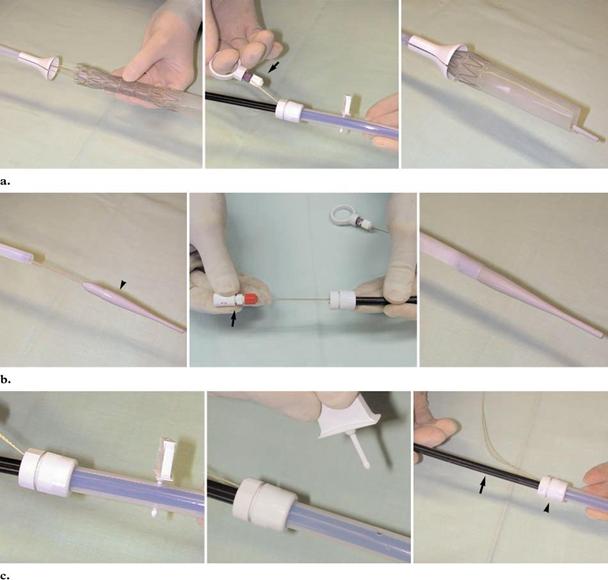
Figure 4. Photographs illustrate the delivery system of the Z-stent. (a) The stent is lubricated and is loaded
through a funnel in the tip of the outer tube by pulling on threads (arrow). (b) The guiding tip (arrowhead) is
adapted to the sheath by pulling on the other end of its shaft (arrow). (c) To deploy the stent, the locking device is
pulled out and the inner catheter (arrow) is held stationary while the outer sheath (arrowhead) is withdrawn. The
thread is cut and removed before retrieving the delivery system.
when gastric pressure rises above a threshold,
thereby allowing patients to belch or vomit (7).
The Z-stent has a higher migration rate when
placed across the gastroesophageal junction (6).
Table 2 summarizes the strengths and weak-
nesses of the various FDA-approved esophageal
stents.
![]()
|