ALTE DOCUMENTE
|
||||||||||
3. Despre polielectroliti
Définition des polyélectrolytes
Un polyélectrolyte est un polymère porteur de charges électriques, et qui est, de ce fait, généralement soluble dans l'eau. A polyelectrolyte is a polymer which carries electric charges, and it is due to that, generally soluble in water. Les polyélectrolytes sont largement présents dans la nature (ADN, polysaccharides , mais aussi au niveau industriel (peintures, gels super absorbants,..).The polyelectrolytes are present in nature in a large amounts (DNA, polysaccharides, etc.), but also at industrial level (paints, super adsorbant gels, etc.). La compréhension des interactions de ces polymères avec d'autres substances chargées est un sujet d'actualité tant au niveau du fonctionnement des systèmes biologiques que de l'élaboration de matériaux à propriétés spécifiques.The understanding of the interactions of these polymers with other charged substances is a subject of present interest at the level of functioning of biological systems as in the elaboration of materials with specific properties. Les polyélectrolytes peuvent facilement se complexer avec des petites molécules tels que les surfactants et avec des macromolécules tels que les protéines.The polyelectrolyte can give complexe substances with small molecules, as surfactants, and with macromolecules, as proteins. Cette complexité conduit à différents types d'organisation moléculaires et supramoléculaires. This complaxation leads to different types of molecular and supramolecular structures.
Un polyélectrolyte est un polymère soluble dans l'eau. Ceci est utilisé pour:
A polyelectrolyte is a polymer soluble in water. For that reason it is used for:
- &nbs 24124r1715y p; &nbs 24124r1715y p; Le développement des procédés "propres" qui remplace les solvants organiques des procédés classiques par des milieux aqueux,
- &nbs 24124r1715y p; &nbs 24124r1715y p; Developing « peculiar » method which replace the organic solvents used in clasical methods with the aqueous media,
- &nbs 24124r1715y p; &nbs 24124r1715y p; dans le traitement de l'eau,
- &nbs 24124r1715y p; &nbs 24124r1715y p; wastewater treatment,
- &nbs 24124r1715y p; &nbs 24124r1715y p; apporter au produit final des propriétés d'usage variées: agent stabilisant des mousses pour les produits cosmétiques, épaississants et gélifiants des produits alimentaires, rhéofuidification des peintures, stabilisants des suspensions colloïdales.
- &nbs 24124r1715y p; &nbs 24124r1715y p; Confering to the final product multiple application properties : stabilizing agent of foams for the cosmetic products, thickening agents and gelifiers of food products, rheofluidization of paints, stabilizers of colloidal suspensions.
Un polyélectrolyte est un réservoir de charges électrostatiques. Il résulte :
A polyelectrolyte is a reservoir of electrostatic charges. It results:
- &nbs 24124r1715y p; &nbs 24124r1715y p; un caractère extraordinairement hygroscopique - les supers absorbants sont des gels de polyélectrolyte,
- &nbs 24124r1715y p; &nbs 24124r1715y p; a extrordinary hygroscopic character - the super absorbants are polyelectrolyte gels,
- &nbs 24124r1715y p; &nbs 24124r1715y p; qu'on peut agir sur les polyélectrolytes avec un champ électrique et on utilise ceux ci en électrophorèse pour séparer des macromolécules en fonction de leur charge.
- &nbs 24124r1715y p; &nbs 24124r1715y p; That it can be acted on a polyelectrolyte with an electric field and this can be used in an electrophoresis for the separation of macromolecules according to their charge.
Les chercheurs développent des polyélectrolytes amphiphiles qui adoptent des comportements associatifs qui leur confèrent des propriétés d'usage intéressantes. Researchers developed amphiphile polyelectrolytes which assume an associative behaviour which confer them interesting utilization properties. Les polyélectrolytes amphiphiles sont composés de deux sous unités: une ou plusieurs portions polyélectrolyte associées à des portions neutres hydrophobes (voir figure 1.1).The amphiphile polyelectrolytes are composed of two subunits: one or more polyelectrolyte's partitions associated with hydrophobic neutral partitions. On parle de:
It may be discussed of:
- &nbs 24124r1715y p; &nbs 24124r1715y p; copolymère à blocs, lorsque ces sous unités s'enchaînent sous forme de bloc,
- &nbs 24124r1715y p; &nbs 24124r1715y p; Bloc-copolymers, because their subunits are chained assume the shape of a bloc,
- &nbs 24124r1715y p; &nbs 24124r1715y p; de polyélectrolyte modifié hydrophobe, lorsque des chaînes hydrophobes sont greffées sur un squelette de polyélectrolyte.
- &nbs 24124r1715y p; &nbs 24124r1715y p; Hydrophobic modifyed polyelectrolytes, because the hydrophobic chaines are grafted on a polyelectrolyte's skeleton.
Dans les deux cas, les parties hydrophobes s'associent par "effet hydrophobe" pour diminuer l'aire des parties hydrophobes en contact avec l'eau en forme de: micelle, cylindre avec un coeur hydrophobe ou sous forme lamellaire. In both cases, the hydrophobic parts associate due to an "hydrophobic effect" to diminish the air formed as the hydrophobic parts make contact with water, in the form of: micelle, cylinder with a hydrophobic core or in lamellar shape.

Figure 1.1 - Les polyélectrolytes diblocs hydrophobes sont constitués d'un bloc polyélectrolyte et d'un bloc hydrophobe neutre qui forment en solution des assemblages supramoléculaires variés: micelles, cylindres, phases lamellaires et vésicules [2].
The dibloc hydrophobic polyelectreolytes are composed of a bloc polyelectrolyte and a neutral hydrophobic bloc which form in solution different supramolecular aggregates: micelles, cylinders, lamellar phases and vesicles.
Les polyélectrolytes interagissent fortement avec les objets de charge opposée. Les possibilités de mélanges sont: polyélectrolyte - surfactant, polyélectrolyte - polyélectrolyte, polyélectrolyte - protéine, polyélectrolyte - colloïde, polyélectrolyte - liposome et polyélectrolyte - cellule vivante. Dans tous les cas, les systèmes ont une tendance à former des assemblages plus ou moins bien définis et la compréhension précise de ceux-ci est souvent difficile.The polyelectrolytes strongly interact with the bodies of opposite charge. The mixing possibilities are: polyelectrolyte-surfactant, polyelectrolyte-polyelectrolyte, polyelectrolyte-protein, polyelectrolyte-colloid, polyelectrolyte-liposome, polyelectrolyte-living cell. In all the cases, the systems have a tendency to form aggregates more or less defines and their total understanding is quite difficult.
Le polyélectrolyte est une macromolécule contenant une fraction f non nulle de monomères ionisables. The polyelectrolyte is a macromolecule containing a fraction, f, not nule of ionisable monomers. On en représente comme un enchaînement de N monomères répartis en (1-f)N monomères neutres notés M et de fN monomères ionisables notés A- / B+, susceptibles d'être dissociés en A- et B+ (voir figure 1.3). It is represented as a concatenation of n monomers distributed in (1-f)N neutral monomers denotes M and fN ionisable monomers denoted A-/ B+, capable of dissociating in A- and B+ (see figure 1.3). M et A- / B+ s'enchaînent aléatoirement et deux paramètres suffisent à décrire un polyélectrolyte :M and A-/B+ concatenate aleatory and two parameters are enough to describe a polyelectrolyte:
- &nbs 24124r1715y p; &nbs 24124r1715y p; N - longueur de la chaîne, nombre de monomères par chaîne,
- &nbs 24124r1715y p; &nbs 24124r1715y p; N- lenght of the chain, number of monomers per chain,
- &nbs 24124r1715y p; &nbs 24124r1715y p; f - taux de charge chimique, avec 0 < f
- &nbs 24124r1715y p; &nbs 24124r1715y p; F- raport, indice, grad, coeficient of electric charge, with 0<f<1.
Si l'enchaînement des monomères ionisables le long de la chaîne se fait par les parties A-, on parle de polyélectrolyte anionique (voir figure 1.3).If the concatenation of ionisable monomers along the chain is made throu partitions A-, we speak about anionic polyelectrolyte ( see figure 1.3). Lorsque les charges se dissocient: When the charges dissociate:
- &nbs 24124r1715y p; &nbs 24124r1715y p; il se forme un polymère de charge négative, appelé polyanion, et un grand nombre de cations simples, les contrions, se dispersent dans la solution,
- &nbs 24124r1715y p; &nbs 24124r1715y p; it is formed a polymer with megative charge, called polyanion, and a great number of cations, the counterions, dispersed in solution,
- &nbs 24124r1715y p; &nbs 24124r1715y p; il se forme un polymère de charge positive, appelé polycation, et un grand nombre d'anions simples, les contrions, se dispersent dans la solution.
- &nbs 24124r1715y p; &nbs 24124r1715y p; It is formed a polymer of positive charge, called polycation, and a great number of simple anions, the counterions, dispersed in solution.
A partir de cette définition, on distingue plusieurs classes de polyélectrolytes. From this definition, we distinguish few classes of polyelectrolytes.
Lorsque f est faible, la présence de monomères ionisables, éventuellement dissociés, constitue une faible perturbation par rapport au cas neutre. When f is small, the presence of ionisable monomers, eventually dissociated, constitutes a weak perturbation compared with the neutral case. Forces de Van der Waals et électrostatiques sont en compétition. On parle de : Van der Waals and electrostatic forces are competing. We can talk about:
- &nbs 24124r1715y p; &nbs 24124r1715y p; polyélectrolyte faiblement chargé, lorsque le taux de charge f est suffisamment petit pour que les forces de Van der Waals soient prépondérantes,
- &nbs 24124r1715y p; &nbs 24124r1715y p; weakly charged polyelectrolyte, when the f is sufficiently small because the Van der Waals forces are preponderent,
- &nbs 24124r1715y p; &nbs 24124r1715y p; polyélectrolyte fortement chargé, lorsque le taux de charge f est suffisamment important pour que les forces d'origine électrostatique soient prépondérantes,
- &nbs 24124r1715y p; &nbs 24124r1715y p; strongly charged polyelectrolyte, when the f is sufficiently large because the electrostatic forces are preponderent.
Les polyélectrolytes faibles les plus courants sont des homopolymères constitués de monomères possédant une fonction acide faible ou base faible. The weak polyelectrolytes , most important the homopolymers constituted from monomers possessing a weak acidic or basic group.
Si la forme : If the :
- &nbs 24124r1715y p; &nbs 24124r1715y p; basique portée par le polymère est chargée, le polyélectrolyte est anionique et l'affinité pour les protons se neutralise à mesure que le pH décroît.
- &nbs 24124r1715y p; &nbs 24124r1715y p; Basic group of the polymer is charges, the polyelectrolyte is anionic and the affinity for protons is neutralised as the pH decreases.
- &nbs 24124r1715y p; &nbs 24124r1715y p; acide portée par le polymère est chargée, le polyélectrolyte est cationique et d'autant plus dissocié que le pH est faible et cède des protons et se neutralise lorsque le pH augmente:
- &nbs 24124r1715y p; &nbs 24124r1715y p; acid group of the polymer is charged, the polymer is anionic and dissociates as long as the pH is small and donates protons and is neutralised when the pH increasses.
Les polyélectrolytes forts ont le taux de charge indépendant de la composition du milieu. The strong polyelectrolytes have the f independent of the media composition. Les polyélectrolytes forts sont le plus souvent composés de monomères portant un groupe acide fort qui se dissocie totalement en libérant H+ ou des espèces salines de réactivité chimique nulle. The strong polyelectrolytes are usually composed of monomers carrying a strong acidic group which totally dissociates freeing H+ or alkaline species of nule chemical reactivity.
Il est important de remarquer qu'un polymère n'est pas intrinsèquement ionomère ou polyélectrolyte. It is important to notice that a polymer is not an intrinseque ionomer or polyelectrolyte. Seule l'interaction entre le solvant et le polymère va déterminer un comportement ionomère ou polyélectrolyte. Only the interaction between solvent and polymer will determine an ionomer behaviour or polyelectrolyte. Sachant que l'eau est le solvant le plus courant des polyélectrolytes, on distingue deux classes de polyélectrolytes :When water is used as solvent, we distinguish two classes of polyelectrolytes:
- &nbs 24124r1715y p; &nbs 24124r1715y p; polyélectrolyte hydrophile: le polyélectrolyte est en bon solvant dans l'eau.
- &nbs 24124r1715y p; &nbs 24124r1715y p; Hydrophile polyelectrolyte : the polyelectrolyte is a well solvated in water.
- &nbs 24124r1715y p; &nbs 24124r1715y p; polyélectrolyte hydrophobe : le polyélectrolyte est en mauvais solvant dans l'eau.
- &nbs 24124r1715y p; &nbs 24124r1715y p; Hydrophobic polyelectrolyte : the polyelectrolyte is not soluble in water.
Cette notion peut être généralisée dans le cas de polyélectrolytes solubles en solvant polaire différente de l'eau. This notion may be generalized in the case of polyelectrolytes soluble in polar solvents different from water. On parle alors de:Then we have:
Les précédents modèles considèrent uniquement les répulsions électrostatiques dues aux charges portées par le macroion et ne considèrent pas le rôle des contre ions. The previous models consider only the electrostatic repulsions due to the charges of the macroion and not taking into consideration the role of the counter-ions. Or ceux-ci peuvent neutraliser certaines charges. Those can neutralize some charges. Ils peuvent se dissocier totalement du macroion (contre ions libres) et gagner de l'entropie en solution, ou bien rester associer à une chaîne (condensation), ce qui est favorable énergétiquement mais défavorable entropiquement. They may totally dissociate from the macroion ( free counter-ions) and gain the entropy in solution, or may remain associated to a chain ( condensation), which is energetically favorable but enthropically unfavorable.
On considère une solution de chaînes polyelectrolytes infiniment longues et toutes parallèles. Consider a solution of polyelectrolyte's chains infinitely long and all parallel. Autour des chaînes, il existe une surface équipotentielle où le potentiel électrostatique s'annule. Along those chains, there is an equipotential surface where the electrostatic potential is cancelled. Cette surface peut être approximée par un cylindre parallèle à la chaîne et d'épaisseur R. This surface may be approximated with a cylinder parallel to the chain and of thickness R. D'un point de vue électrostatique, chaque chaîne avec ses contres ions contenus dans le cylindre de rayon R, est indépendante des autres chaînes. From electrostatic point of view, each chain with its counter-ions contained in the cylinder of radius R, is independent of the other cahins. C'est donc un modèle de cellule avec une condition aux limites annulant le potentiel électrostatique à la distance R. It is a cellular model with boundary conditions canceling the electrostatic potential at distance R. Dans cette approche, la distribution des contres ions est continue et il n'y a pas d'état condensé proprement dit. In this approach, the counter-ion's distribution is continued and there is no condensation. L'espace se divise en deux régions : the space is divided in two regions:
- &nbs 24124r1715y p; &nbs 24124r1715y p; une première région où l'énergie d'interaction entre les contres ions et supérieure à kT et où les contres ions sont donc liés à la chaîne ;
- &nbs 24124r1715y p; &nbs 24124r1715y p; A first region where the energy of interaction between the counter-ions is higher then kT and where the counter-ions are tied to the chain,
- &nbs 24124r1715y p; &nbs 24124r1715y p; une seconde région, plus loin de la chaîne où l'énergie d'interaction entre les contre ions et la chaîne est inférieure à kT et où les contre ions ne sont donc plus associés à la chaîne.
- &nbs 24124r1715y p; &nbs 24124r1715y p; A second region, further on the chain where the energy of interaction between the conter-ions and the chain is smaller then kT and where the counter-ions are no longer attached to the chain.
L'étude des interactions entre micelles est très importante dans ce régime pour la modélisation du comportement des solutions colloïdales protégées par des polyelectrolytes.
Pour les étoile neutres aucun ordre entre objet n'a été observé avant c*. A c* un maximum d'ordre est observé et interprété comme un maximum de la pression osmotique Ensuite, au de la de c*, l'ordre disparaît, ce qui est interprété comme une interpénétration des micelles entre elles. Pour une micelle de forte densité (p ), un ordre apparaît bien à c* et persiste au-delà de c*. L'existence de cet ordre à de si hautes concentrations est incompatible avec une interpénétration des couronnes. L'interprétation des auteurs est une concentration des couronnes sans interpénétration de celle-ci. On cherche à étendre cette étude à des couronnes de plus faibles densités. Il semble raisonnable de penser que la densité de la couronne aura effet sur le comportement des micelles au-delà de c*. En effet, à cause de l'extension radiale des bras chargés, les couronnes peu denses pourront s'interpénétrer, sans changer la conformation de leur bras, c'est-à-dire sans apparition d'interactions entre bras : à la périphérie la concentration en polymère sera toujours faible. Il est aussi raisonnable de penser que, pour des nombres des bras intermédiaires, une interpénétration des couronnes peut avoir lieu comme pour les brosses sphériques neutres.
I.A.2. POLYMERS AND POLYELECTROLYTES
Polymers are molecules composed of a big number of key- units (N=10^@ to 10^6), called "monomers". When those monomers are charged, we refer to as polyelectrolytes. In pure state, the polymers are used for the manufacture of common objects as bottles for water (polyethylene terephtalate), polycarbonated glass, joints (siliconed polymers), tuyaux (PVC), or pare-chocs (polypropylene).In aqueous solution, polymers and polyelectrolytes are used for their rheologic properties as thickening agents and gelifiers, which makes them very useful in the cosmetics formulae or agro-alimentary. Polyelectrolytes are used as well for their adsorption properties at interfaces. The cationic polyelectrolytes are used in the shampoo formulae, because it can adsorb in to the hair and fortify it. We can assume that our body itself is composed of numerous polyelectrolytes molecules, as long as our DNA and all the proteins which compose our cells are also charged long-chain molecules.
Polyelectrolytes
Like repulsions between the charged monomers, the polyelectrolytes have a longuers de persistence much more higher then the neutral polymers. This results from the fact that the chains are more rigid and more expanded in space. Also, it requires low concentrations of polyelectrolyte because it is framed in the semi-diluted regime(medium).
Are distinguished strong polyelectrolytes, who's charges are fixed on the chain, like SO3- groups, weak polyelectrolytes, who's charges depend on the pH (-COOH groups). The charged monomer fraction is another key parameter which defines the polyelectrolyte.
Manning condensation
The counter-ions of a polyelectrolyte chain can self-condense on the chain, which is energetically favorable, or are dissociating in solution which is entropically favorable. The ions which are participating to condensation also allow screening the repulsions between the charged monomers. From this equilibrium, it results that the distance between two charges which dissociate successively is equal to the Bjerrum length which measures 7 A in water. For a vinylic chain, the minimal distance between two ionisable monomers is 2.5 A. If all monomers are ionisable, only a monomer of three is effectively charged so the 7 A length between two charges can be abided. It results that the effective charge ratio of a polyelectrolyte can not exceed 33% ionisable monomer content.
Structure in diluted and semi-diluted regimes
We can write the free energy of a polyelectrolyte as the sum of the component determined by electrostatic repulsions between monomers and the elastic component. Minimizing this energy proportionally to R, we find that the ray of chain varies linearly with the number of N monomers and increases exponentially with the effective charge ratio of the polyelectrolyte.
The similitude with the lather law allows to show
that the chain in diluted solution constitutes electrostatic circles(globules) of diameter,![]() . which corresponds
to the length for which the thermic agitation energy (kT) and the electrostatic
energy compensate each other. Inside a circle the chain has an ideal
conformation. For the chains with higher molecular weight, the circles grow
electrostatically and have the tendency to line up like a stick (Figure
I-17-a).
. which corresponds
to the length for which the thermic agitation energy (kT) and the electrostatic
energy compensate each other. Inside a circle the chain has an ideal
conformation. For the chains with higher molecular weight, the circles grow
electrostatically and have the tendency to line up like a stick (Figure
I-17-a).
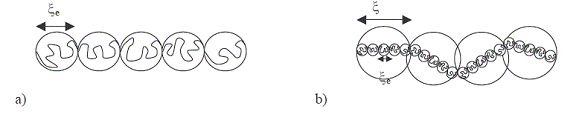
Figure I-17: a- stick(batonnete) formed
from electrostatic circles of dimension,![]() representing a polyelectrolyte chain in diluted solution; b- inlantuire
of circles of dimension
representing a polyelectrolyte chain in diluted solution; b- inlantuire
of circles of dimension![]() , containing electrostatic circles of
dimension
, containing electrostatic circles of
dimension![]() .
.
After a concentration c*, we enter
the semi-diluted regime and the sticks start to overlap. The solution is then
composed of circles of dimension![]() , containing smaller circles of dimension
, containing smaller circles of dimension![]() .
.
Note that the viscosity of semi-diluted solutions of polyelectrolytes increases with the charge ratio or with the length of the polyelectrolyte and decreases with the concentration of salt. Indubitable, the salt unscreens(blurs, blocks) the repulsions between charged monomers, which determines the collapse(breakdown) of the chain.
Polyelectrolytes in weak solvent
Different regimes have been studied, function of the salt concentration and the charge ratio of polyelectrolyte. The salt had the effect of modifying at the same time the interaction between the counter-ion and the monomer, and also the interactions between the charged monomers, the chains being able to adopt three different configurations: besides the pearls necklace configuration, the chains can assume the shape of globules, of "sausages", or they can maintain a stretched configuration.
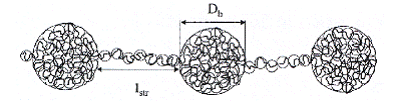
Figure I-18: Pearls necklace formed in solution by a chain of a hydrophobe polyelectrolyte
I.A.2.b. At interfaces
The adsorption of polymers and polyelectrolytes is of great practical interest: for example, a layer of adsorbed polymer can help decreasing the adhesion between two surfaces (coating a pan with Teflon), to stabilize the colloidal dispersions from steric repulsions between adsorbed chains (stabilization of cosmetic emulsions), or on the contrary for unstabilizing a dispersion (flocculation of polluting particles "by creating a bridge" for water treatment). So it is important to understand why and how the polymers adsorb themselves at interfaces. The case of polyelectrolyte is a lot more complex due to the fact that the short range forces, the effects of counter-ions and the multitude of parameters and interaction come out in the game.
The adsorption of polyelectrolytes at interfaces is a complex phenomenon, which depends on the charge of the surface, on the polyelectrolyte and on the salt concentration.
Generally, if the surface and the chains are of opposite charge, it exists adsorption. Otherwise, if the chains and the surface are of the same charge, they have the tendency to repulse themselves. In the case of a uncharged surface, there is a small attraction of the polyelectrolyte towards the surface, if it exists, of course, a chemical affinity between the monomers and the surface. For example, the hydrophobe polyelectrolytes have the tendency to adsorb at interface water-air and water-oil. The principal results concerning the adsorption of polyelectrolytes on a surface of opposite charge shows that the attraction of chains for the surface is screened by the presence of salt. More over, while the charge of the surface increases, the chain forms first of all a diluted medium (regime) then semi-diluted of two dimensions. Then, the chains start forming a self-similar grill of three dimensions, with tails which spread in the solution. When the solution becomes too charged, it becomes too constrained entropically for the chains to adsorb more monomers and some sites are replaced by counter-ions from the surface, which screen the attraction of chains for the surface. It results that the chains are separated from more monomers from the surface and that the thickness of the layer increases.
At weak ionic forces, the adsorption of polyelectrolytes over an uncharged surface is small because the chains don't exhibit affinity towards the surface and are good solvent in water. At high concentrations of salt, the chains are collapsing by themselves and can self-adsorb.
Adsorption of hydrophobe polyelectrolytes
There are many types of hydrphobe polyelectrolytes: The synthetic polyelectrolytes like the statistique copolymers or di- and tri- blocks, like modified polyelectrolytes with permanently hydrophobic tails. The diblocks and the triblocks are composed of charged monomers and of hydrophobe monomers. They can form micelles in solution. In the case of modified polyelectrolytes with permanently hydrophobic tails, the tails can form associations in solution and start (inbreed, engender) a network capable of thickening the solution. In a general way, the hydrophobe polyelectrolytes can self-adsorb at interface water-air if the number of hydrophobic entities is sufficient and the charge ratio is moderate. Based on the resemblance of these systems with our complexes polyelectrolyte-surfactant, we will describe the adsorption of the multiblocks copolymers and statistique copolymers.
Adsorption of the statistique "copolyelectrolytes"
Theory
As we've seen, the hydrophobe polyelectrolytes can form pearls "necklaces" and also globules in solution. These structures, more over the globules, are favorised at small charges ratio or at higher ionic force, because the electrostatic repulsions between chains are screened and the attractions between hydrophobe monomers become important.
Borisov showed that the adsorption of globules and pearl "necklaces" over a opposite charged surface is controlled by a balance between the electrostatic attraction of the chain towards the surface, the electrostatic repulsion between charged monomers and the surface energy of globules and pearls. While the ionic force increases or if the charge of the surface is weak, the globules are little attracted by the surface and remain spherical to minimize their surface energy. In certain cases, they can also disadsorb. If the surface is too charged, the globules squash (flatten) over the surface and form a pie-shape (Figure I-23).
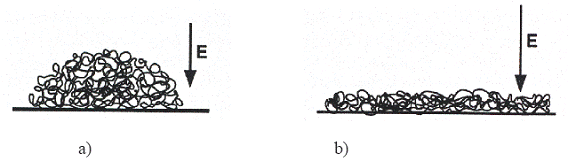
Figure I-23: a- adsorption of a globule of hydrophobe polyelectrolyte over an opposite charged surface. While the charge of the surface is weak the globule remains spherique. b- while the charge is higher the globule flattens itself to form a pancake.
In the case of pearls necklace, the pearls can also flatten if the surface is too charged. While the ionic force increases, these flattened pearls can coalescer and also desorber.
|