THE ACTIVITY OF THE ERYTHROCYTES CARBONIC ANHYDRASE II USED AS A TUMORAL MARKER IN DIGESTIVE CANCER PATIENTS
INTRODUCTION
Discovered in 1932 by Meldrum and Roughton , cabonic anhydrase (CA) forms a zinc metalloenzymes family whose basic physiological role is to catalyse the interconversion of carbon dioxide and bicarbonate (CO2+H2O H+ + HCO3-) and takes part in various physiological processes that involve pH regulation, HCO3- and CO2 transport, ion transport and electrolyte balance. The functions which depend directly or indirectly on CA are H+ secretion and HCO3- reabsorption in order to maintain the acid-base equilibrium. The CA isozymes are found in almost all mammalian tissues . They are located in red blood cells and in vascular walls (CAI) are cytoplasmic (CA II, III and VII), membrane-bound (CA IV), mitochondrial (CA V), and salivary (CA VI). The CA II isozyme, well-known for its high activity is present in large amounts in erythrocytes and is the most widely distributed isozyme of the CA family.
As concerns the physiological functions of CA, our studies have proven that isozyme I - which is located in red blood cells and also in vascular walls - is involved in the modulation of vascular processes. Isozyme II of CA, found both in red blood cells and in secretory cells, modulates secretory processes. In the same studies we have established that by causing pH changes carbonic anhydrase is involved in the modulation of physiological and pathological processes in organisms and hence that alkaline intracellular pH may play a significant role in carcinogenesis.
Studies carried out by our team have proven that anticarcinogenic drugs activate CA II, while carcinogenic substances decrease the activity of this isozyme .
Our previous studies have shown that the serum of gastric, esophageal, hepatic, pancreatic and colorectal carcinoma patients explosively activates purified CA II activity.
AIM
In this paper we followed-up the red blood cells CA II activity in cancer patients having different primary locations.
MATERIAL AND METHOD
The experiments were approved by the local human ethics committee and informed consent was obtained from each patient. We selected 5 groups of digestive cancer patients in different stages, histologically confirmed and another 2 groups, of healthy volunteers and of patients with non-cancer diseases, as follows:
Group 1 (N=259) - gastric cancer, types: lymphoma (N=131), polypoid type I (N=96) and depressed type IIc (N=32);
Group 2 (N=214) - esophageal cancer, types: squamous cell carcinoma (N=97), adenoid cystic carcinoma (N=71) and primary esophageal lymphoma (N=46) ;
Group 3 (N=207) - colorectal cancer: stage A (limited to mucosa) (N=85) and stage B2 (through serosa) (N=122);
Group 4 (N=203) -hepatic cancer, types: hepatocellular carcinoma (N=79), cholangiocarcinoma (N=63), hepatoblastoma (N=47) and angiosarcoma (N=14);
Group 5 (N=211) - pancreatic cancer, types: adenosquamous carcinoma (N=73); lymphoma (N=81), sarcomatoid carcinomas: spindle cell carcinoma (N=15), malignant giant cell carcinoma (N=14), pleomorphic giant cell carcinoma (N=11) and round cell anaplastic carcinoma (N=7).
Group 6 (N=219) - patients with other non-cancer diseases of the same organs;
Group 7 (N=255) - healthy volunteers.
Blood was collected by venous puncture and 50µl were dropped in 4.95 ml twice distilled water. In this way hemolysis was achieved and the dilution of the hemolysate was 1/100. In all patients hemoglobin blood content was spectrophoto-metrically assessed as CN Met-Hb at 540 nm. The final blood dilution in the reaction mixture for the assays of CA activity was 1/2000.
CA activity was assessed by the
stopped-flow method . This consists of measuring the
enzymatic activity of CO2 hydration and it relies on a colorimetric
method of assaying changes in pH. The time in which the pH of the reagent
mixture decreases from its initial value of 7.5 to its final value of 6.5 is
spectrophotometrically observed at 400 nm wave length, using a rapid kinetic
spectrophotometer HI-TECH SF-51MX (
Reagents used:
- p-nitrophenol - as color indicator, is used at a concentration of 0.2 mM; pH=7.5; temperature = 20-25oC.
- HEPES buffer at concentration of 20 mM; pH=7.5; r.t 20-25oC.
- The CO2 solution at concentration of 15 mM (as substrate) which is obtained by bubbling CO2 in bidistilled water to saturation.
- Na2SO4 at concentration of 0.1 M is used to keep up constant ionic strength.
Activity of carbonic anhydrase is obtained by the formula:
A = T0-T x dilution x 1 [EU/ml]
T gHb
where T0 represents the uncatalyzed reaction time;
T represents the catalyzed reaction time (in the presence of red blood cell CA).
g Hb - concentration of hemoglobin.
Differentiation of red cell CA I from CA II activity was carried out by means of the nicotinate test, substance which completely inhibits red cell CA I activity and does not significantly modify the CA II activity.
Statistical processing of data was accomplished by Student's t-test.
RESULTS
Our data show that erythrocyte CA II activity is significantly lower in cancer patients irrespective of site of tumor origin, as compared to the activity of the same isozyme in control a group or in the patients having diseases other than cancer (Table1).
Table 1. The values of erythrocyte CA II activity in cancer patients,
healthy volunteers and patients with non-cancer diseases.
Data are presented as means S.E.; n = 171-259 patients
|
Group |
Diagnosis |
CA II activity (E.U./gHb) |
|
Gastric cancer | ||
|
Esophageal cancer | ||
|
Colorectal cancer | ||
|
Hepatic cancer | ||
|
Pancreatic cancer | ||
|
Non-cancer diseases | ||
|
Healthy volunteers |
* significant differences (p<0.05) as compared with healthy volunteers .
For instance, in the digestive cancer patients, mean of erythrocyte CA II activity is 79.60±7.29 EU/g Hb, as compared to the control group activity of 189.76±10.23 EU/gHb , while the non-cancer patients activity of the same enzyme was 173.21±9.12 (Fig.1).
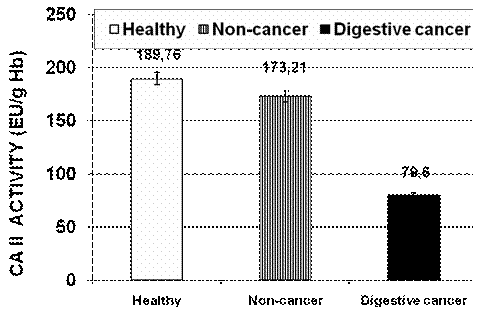
Fig.1. The values of erythrocyte CA II activity in digestive cancer patients as compared to healthy volunteers and patients with non-cancer diseases .
DISCUSSIONS
As is known, CA II is a cytosol enzyme, having a major role in maintaining the acid-base balance. This isozyme with a high catalytic activity seems to be one of the most widely distributed enzymes in organisms being located in all cells' cytoplasm and in ertythrocytes. Erytrhocytes contain a sufficient amount of high-activity CA II to ensure the hydration and CO2/HCO3- transport processes. Human red blood cells contain 85% CA I - a low-activity enzyme and 15% CA II which is a high-activity enzyme and therefore human red blood cells represent the main source of CA.
The presence of CA II in erythrocytes has enabled us to study this enzyme in detail i.e. to study its activators and inhibitors, as well as the therapeutic effects of some pharmacological agents.
Our in vitro and in vivo studies have proven for the first time that anticarcinogenic drugs like cyclophosphamide increase erythocyte CA II activity whereas carcinogenic substances such as benzpyrene or acetamidofluorene reduce this activity.
The results of the present study confirm that erythrocytes' CA II actvitiy is low in a broad range of cancer types. Our other research work show a direct relationship between CA II activity and the tumor stage. Thus, erythrocyte CA II activity falls as the neoplasic process advances.
Our results suggest that the change of intracellular pH may play a role in malignant transformation by inhibiting control of pH balance. Reducing CA II activity is followed by a rise in intracellular pH, leading to cellular alkalization, a process described by Stubbs in his study of carcinogenesis .
Our results should encourage future studies focused on the pH changes initiated by cytosolic CA II-induced inhibition including the influence of such changes on control of cell growth, transformation and the handing of carcinogenic substances.
CONCLUSIONS
1. Erythrocyte CA II activity in cancer patients with a broad range of tumor types significantly lower than controls' values in healthy patients or the isozyme values in patients with other diseases.
2. The study of erythrocyte CA II activity suggests that one possible loss of control of intracellular pH changes may lead to carcinogenesis.
Carter M.J.: Carbonic anhydrase: isoenzymes, properties, distribution and functional significance. Biol.rev., 47:465-513, 1972.
Khalifah R.G. : The carbon dioxide hydration activity of carbonic anhydrase: stop-flow kinetic studies on the native human isozymes B and C. J. Biol. Chem., 246:2561-2573, 1971.
Lonnerholm, G., Wistrand, P.J.: Amount and distribution of carbonic anhydrases I and II in the gastrointestinal tract. Gastroenterology, 88:1151-1161, 1985.
Maren T.H.:Carbonic anhydrase: chemistry, physiology and inhibition. Physiol.Rev., 1967, 595-748.
Meldrum N.U. , Roughton J.W. : Carbonic anhydrase:its preparation and
properties. J.Physiol. (
Puscas, C.,
Puscas,
Puscas, I. et al:The serum of gastric carcinoma patients explosively
activates purified carbonic anhydrase II activity as compared to controls.
Method of rapid diagnose of gastric cancer. Digestive Disease Week,
Puscas,
Puscas,
Puscas,
Puscas,
Sly, W.S Hu P.Y. : Human carbonic anhydrases and carbonic anhydrases deficiencies. Annu.Rev.Biochem., 64:375-401, 1995.
Stubbs, M.: Tumor pH. The Lancet, 340, 342-346, 1992.
Tashian, R.E., Hewett-Emmett, D., Goodman, M.: Diversity and evolution in the carbonic anhydrase gene family. In: carbonic Anhydrase.From Biochemistry and Genetics to Physiology and Clinical Medicine, Botre F., Gros G, Storey B.T. (eds), VCH, Weinheim, p.151-161, 1991.
Whitney, P.L., Briggle, T.V. : Membrane-associated carbonic anhydrase purified from bovine lung. J.Biol.Chem, 257:12056-12059, 1982.
|