NEW TECHNOLOGIES FOR NITRITE AND NITRATE REMOVAL FROM DRINKING WATER ENVISAGING THE DIMINUTION OF HUMAN HEALTH RISK
Micu Daniela*, Sonea Daniela**, Lemic Jovan***, Ratiu Cornelia****, Manea Florica **, Burtica Georgeta **
ABSTRACT
The risk of nitrite and nitrate ingestion on human health comes from various sources, e.g., water, vegetables and meat produces. Nevertheless the maximum allowance concentration for nitrite and nitrate in drinking water is the smallest in comparison with vegetables and meat products, however water mainly contributes to human health risk because of the great exposure of the population to water ingestion.
The treatment of natural zeolite with quaternary amines converted it into suitable compounds for retaining certain anions, i.e., nitrite and nitrate. Two types of organozeolites were used for the removal of nitrite and nitrate anions, namelly DDAC (distearyl-dimethyl-ammonium-chloride), respectively SDBAC (stearyl-dimethyl-benzyl-ammonium-chloride). Two concentrations of quaternary amines, 100 mM and 200 mM were used to modify the zeolite. The organozeolite performances for the removal of nitrite and nitrate from water were determined.
Key words: organozeolite, nitrite, nitrate, human health risk.
INTRODUCTION
Given the pollution of the air, the quality of the water used by the population may be a significant factor that causes diseases. Besides diseases that are caused by different micro-organisms, water may influence the general health of human collectivities also by its contents of mineral elements that take an active part in vital processes 1 3]
Among environmental issues that have an impact on the death of population caused by non-catching diseases, there is the chemical quality of drinking water. In this sense, it is commonly known that water has a very complex chemical composition that includes a large number of dissolved chemical elements. The natural composition of water includes various mineral substances that can also be found in the human body, macro-elements (salts of calcium, magnesium, natrium, potassium, chlorides, etc.), others in very small quantities, micro-elements (iodine, fluoride, chromium, selenium, vanadium, etc.). They are defined as biogenic substances, vital for the functioning of the body. Their excess disturbs this good functioning, by the possible appearance of public health issues. But water may be also polluted by toxic substances, like: nitrates, pesticides, detergents and heavy metals.
The health risks due to the presence of toxic chemical substances are different from the risks of microbiological contamination of drinking water. Without taking this issue too lightly, the chemical constituents from water rarely lead to acute effects, except for a massive accidental chemical pollution of a water source. It is the health problems caused by long and unwanted exposure to substances with cumulative toxic properties: heavy metals, cancer causing substances.
The contamination with nitrates and nitrites of underground water sources is a serious problem in many regions, especially in the rural area. Agriculture has a major contribution to the deterioration of water quality due to the use of fertilizers and pesticides that infiltrate into the ground water. Consequently, water sources often contain toxic chemical compounds, with a risk concerning the health of the population. One can observe high levels of nitrates and other organic and microbiological polluters, in fountain waters.
The increased concentration of
nitrates and nitrites in well waters is also due to the polluting sources
around the well (lack of hygiene, toilets located at reduced distances and
depths of only 4-5 meters, the disposition of animal stables up-hill as related
to the drinking water source, etc.). After heavy rains periods, water
infiltrates into the local sources of drinking water. Another reason of
pollution is the fertilization of soils by nitrate fertilizers. There are in
The various chemical substances dissolved in water may have significant effects on the health of living bodies in general and on human health in particular. A whole series of non-catching diseases are nowadays considered as being caused or favorized by the chemical composition of water, namely: endemic goiter, tooth decay and endemic fluorosis, heart diseases, methemoglobinemia, etc. Inside the mouth, nitrates turn into nitrites (phenomenon that accentuates in the case of small children due to the increased alkalinity of their saliva) and, in the bloodstream, it combines with the hemoglobin, forming methemoglobin. This phenomenon blocks the red cells of the blood and causes "cyanosis", and, in the case of small children, it may even lead to death ("blue child" disease).
Along with the increase of environment nitrate and nitrite concentration, there has been observed an always increasing incidence of chronic intoxications with nitrates at small children (0-7 years old). Moreover, there is a major risk in the presence of nitrates and nitrites in vegetables in the rural area, with the possibility of forming nitroso-amines, substances that have a high cancer and mutant potential
Zeolite minerals have found an increased range of applications in the field of pollution control, based on their remarkable selectivity in adsorption and ion exchange 13]
They are cage like structures
with high exchange cations capacities. Clinoptilolite is the most abundant
naturally occurring zeolite in
As a chemically modified ion exchange agent, there can be used the natural zeolite of the clinoptilolite type, chemically modified as organozeolite, in view to retaining inorganic anions from water. By altering the surface of the clinoptilolite with amines, a hydrophobic surface was obtained, which can retain inorganic anions.
The treatments of clinoptilolite with quaternary amines make them to gain an affinity for inorganic oxyanions. The affinity is more intense with the length of carbon chain of amines[Lemic nd col.].[14 5]
The aim of this study was to remove nitrite and nitrate anions from water by using Serbian zeolite modified with quaternary amines in order to reduce nitrites and nitrates' risk on human health. Two types of quaternary amines were used to modify zeolite so as to become suitable for nitrite and nitrate removal form water, distearyl-dimethyl-ammonium-chloride (DDAC), respectively stearyl-dimethyl-benzyl-ammonium-chloride (SDBAC).
MATERIALS AND METHODS
Starting from this fact, a study was initiated on reducing the loading of the polluted waters with nitrites by retaining them on organozeolites.
The laboratory research used natural zeolite from the NE of Yugoslavia with a content of 80 - 90 % of clinoptiolit and a composition of: 69 % SiO2; 12,76% Al2O3; 1,25% Fe2O3; 2,80% CaO; 0,29% MgO; 0,45% Na2O; 3,13% K2O; 0,0194% MnO; 0,167% TiO2.
In order to increase its affinity
for the inorganic anions, the natural zeolite was modified with quaternary
amine for: SDBAC type stearyl-dimethyl-benzyl-ammonium-chloride) and DDAC type (distearyl-dimethyl-ammonium-chloride). These organozeolites were prepared by the Institute for Technology
of Nuclear and Other Raw Minerals,
Comparative studies to retain the nitrite and nitrate ions on these organozeolites were carried out.
For effected this studies for remove nitrit and nitrate anions on the zeolite modified with quaternary amines, the nitrite and nitrate solitions with concentrations between 0.46 and 552 mg * L-1.
Two grams of each kind of zeolite (SDBAC and DDAC, 100 and 200mmol/dm3) were mixed with 8 ml of nitrite solution. The samples were shacked at 25°C about 24 h,a period shown sufficient for retain the nitrite and nitrate ions on the zeolite modified with amine quaternary. When the time ran out, the suspension were filtrated and analyzed. To determine the nitrite concentration in solutions the Griess spectrophotometric method was used. It was used a GBC Cintra 5 spectrophotometer. The correlation factor for the curve was 0,9996.
This study was carried out to test the capability of zeolite modified with two concentrations of DDAC and SDBAC (100 and 200 mM) to remove nitrate and especial nitrite from water.
In figure 1 are shown the results obtaining after 12 hours of contact from solution with varions concentrations of nitrate and zeolite modified with 100 and 200 mM DDAC.
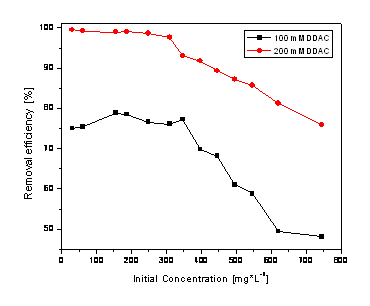
Figure 1. Nitrate removal efficiency using the zeolite modified with DDAC
As we expected the beter results related to the removal efficiency with DDAC with higher concentration (200 mM) was reached for the initial concentration of nitrate up to about 300 mg * dm-3 the removal efficiencies are almost constant and the effiencies decreased beyond this concentration. Using zeolite modified with 100 mM DDAC the efficiency for nitrate removal reached maximum about 75%, and about 99% for 200 mM DDAC.
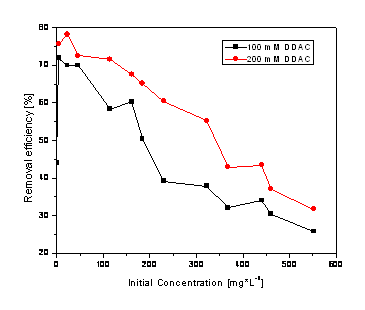
Figure 2. Nitrite removal efficiency using the zeolite modified with DDAC
Figure 2 thows the results obtained for nitrite anion removal under the similar conditions mentioned in figure 1. Also, the higher concentration of quaternary amine led to better results. Though, the efficiencies for nitrite removal over 80 % was not reached.
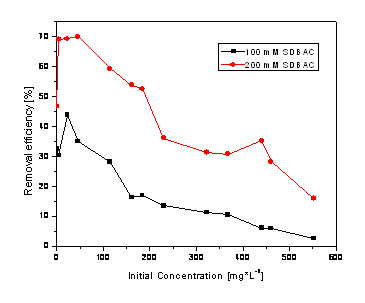
Figure 3. Nitrite removal efficiency using the zeolite modified with SDBAC
In figure 3 the efficiency for nitrite removal using SDBAC is shown. It can be noticed also the better efficiencies for higher concentration of SDBAC.
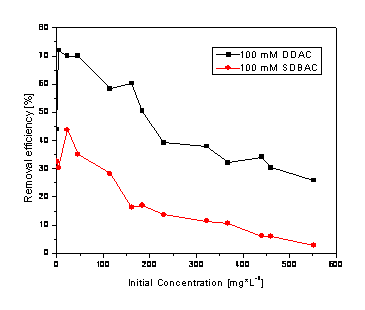
Figure 4. Nitrite removal efficiency using the two types of zeolite modified with 100 mM DDAC and 100 mM SDBAC
Comparing the results obtained for quaternary amines, by using DDAC the better results were reached.
Based on the presented results, it can be concluded that by using 200mM DDAC the very good performance of nitrate and nitrite removal process was achieved.
Modifying zeolite with two types of quaternary amines, e.g., SDBAC stearyl - dimethyl - benzyl - ammonium - chloride) and DDAC (distearyl-dimethyl-ammonium-chloride allowed their using for the removal of nitrite and nitrate anions from water.
The removal process of nitrite and nitrate from water depended on forme factors, i.e., the type of quaternary amine, the concentration of quaternary amine, the initial concentration of nitrate and nitrite ions. Under the studied conditions, the best results subjected to the removal efficiency was reached for using 200mM DDAC. Also, performance of removal process was better for nitrate than nitrite anion.
Acknowledgement
This work was supported by project CEEX-PROAQUA No. 631/03.10.2005.
Vlaicu, B. 1996, Sanatatea mediului ambiant, Ed. Brumar.
[2] Vlaicu B., 1998, Igiena si ecologia mediului, Ed. Eurobit.
[3] Burtica, G., Negrea, A.,
Micu, D., Orha, C., 2005, Polluting Agents and the Environment,
Ed. Politehnica,
Iacob, I., Vasilescu, M., Dragulescu, I., 1999, Riscurile pentru sanatate legate de calitatea apei de baut, Rapoarte si rezultatele lucrarilor, Focsani.
[5] Friptuleac, G., Dobreanski, E., Teaci, E., 1999, Studiul sanatatii populatiei in relatie cu calitatea apei potabile, Rapoarte si rezultatele lucrarilor, Focsani,.
[6] Council Direktive 98/83/EC -Concerning the quality of water intended for human consumption. 3rd November 1998.
[7] Jacqmin, H., Commenges, D., 1996, Components of drinking water and risk of cognitive inpainement in the elderly. Am. Jour. Of Epidemiology, vol.139, nr. 1, 48-57
[8] Kjellstro, T., Rosenstock, L., 1998, The role of environmental health and occupational hazards in the adult health transition World health statistical quarterly 43(3): 195 (1990), World health Statistics Quarterly, vol. 51, No. 1.
[9] Roumain Potable Water Low No. 458/2002 and 311/2004.
[10] Roumain Potable Water Low No. 458/2002.
[11] Radulescu, H., Micu, D., Burtica, G., 2005, Food as a potential source of consumer's nitric stress, Centr.Eur. J. Occup. Environ.Med.:11(2): 87-92
***, www.esr.cri.nz. Barbara Thomson, 2004, Nitrates And Nitrites Dietary Exposure And Risk Assessment, Institute of Environmental Science
& Research Limited,
Cruceanu, M., Popovici, E., Balba, N., Naum, N., Vladescu, L., Russu, R., Vasile, A., 1986, Site moleculare zeolitice, Ed. Stiintifica si Enciclopedica, Bucuresti, p.26
[14] Vujanovic, A., Darkovic, A., Lemic, J., Radoslavljievic-Mihailovic, A., Tomasevic-Canovic, M., 2003, Adsorption of Inorganic Contaminants on Surfactant Modified Minerals. J. Serb. Chem. Soc.68 (11), p. 833-841.
[15] Lemic, J., Tomasevic-Canovic, M., Djuricic, M., Staniuc, T., 2005, Surface modification of sepiolite with quaternary amines, Journal of Colloid and Interface Science.
|